Homework Study Helpers! =P discussion
Science
>
Chemistry
date newest »
newest »
 newest »
newest »
message 1:
by
Andrea
(new)
Apr 23, 2011 09:06PM
 elements and other stuff here :)
elements and other stuff here :)
reply
|
flag
 Im decent at chemistry, though some stuff confuses me, especially the more complex stuff. It can still be fun though :))
Im decent at chemistry, though some stuff confuses me, especially the more complex stuff. It can still be fun though :))
 I actually loved chemistry, though not exactly professional or advanced in it but enough to help I hope :)
I actually loved chemistry, though not exactly professional or advanced in it but enough to help I hope :)
Chemistry is so hard, i had to stay up for hours just so i could pass
 I used to LOVE chemistry when I took a basic course at my old school (every 8th grader would switch science teachers every quarter, so we got to learn the basics of astronomy, physics, and chemistry all in one year). Seriously, I loved it so much and adored my teacher, and I did extra credit even though I had an A+.
I used to LOVE chemistry when I took a basic course at my old school (every 8th grader would switch science teachers every quarter, so we got to learn the basics of astronomy, physics, and chemistry all in one year). Seriously, I loved it so much and adored my teacher, and I did extra credit even though I had an A+. Now this year in 10th grade I'm homeschooled, taking online courses with online teachers. I decided to take the full chemistry course because I thought I would love it but I HATE IT. And I don't think my teacher really likes me. I have a horrible grade and it makes me so sad. :(
dont do online it's stupid and u won't learn alot
 Kassie Julia wrote: "dont do online it's stupid and u won't learn alot"
Kassie Julia wrote: "dont do online it's stupid and u won't learn alot"I did my chemistry through correspondence...similar to online. Only thing that is not good about it is that you have the final exam which was VERY long and you had to study for the whole five units of material...:( I suck at exams...lol No midterm exams...:(
however I did learn quite a few things...Mostly from help of Youtube teachers! lol
 Kaitlyn Analyssa wrote: "i'm really struggling in chemistry because i believe that my teacher makes it harder than it has to be. But for not i want to understand the concept of the whole 2s3p4d concept"
Kaitlyn Analyssa wrote: "i'm really struggling in chemistry because i believe that my teacher makes it harder than it has to be. But for not i want to understand the concept of the whole 2s3p4d concept"Hi Kaitlyn,
So basically the concept of 2s3p4d and I'll use and example of say....Fe Iron. And I'll try to explain with out big words.
You have Iron which is element 26.
As you know electrons orbit around an atom right?
now going further into that...there are different orbitals and each orbital contains a certain amount of electrons.
s orbital which contain only two electrons
p orbital will contain 6 electrons
d orbitals will contain 10 electrons at any time.
f orbitals will hold 14 electrons.
So when you begin to fill the energy level diagram there are rules:
1) Pauli Exclusion Principle ("Opposite Spin")
an electron is believed to spin like a toy top does. so when filling an orbital you use arrows to represent the electron.
2) Aufbau Principle ("lowest Fill First")
when oribals are filled with electrons, orbital swith the lowest energy are filled first.
example the two electrons of helium are found in the 1s orbital.
3) Hund's Rule ( "Align Spins")
before filling the orbital you should make sure each orbital has one electron it in before you start to pair them.

Direction in which to fill an energy level diagram
I wasn't sure what part of the concept you didn't understand so I put up basic stuff...which you probably might already know
 Hmm. I took AP Chem last year and got a 5 on the AP test, so maybe I'll be able to help out in here. Not sure how much I remember, but I'll do my best.
Hmm. I took AP Chem last year and got a 5 on the AP test, so maybe I'll be able to help out in here. Not sure how much I remember, but I'll do my best.Just a quick word of encouragement: If you're not good at chem now, don't worry about it too much. It has a little do to with the way your brain works, and how good you are at thinking about things that aren't concrete. I was terrible at chemistry the first time I encountered it, in eight grade, but totally rocked it two years later. So just 'cause you don't get it now doesn't mean you never will, though it could be a while.
 Anila wrote: "Hmm. I took AP Chem last year and got a 5 on the AP test, so maybe I'll be able to help out in here. Not sure how much I remember, but I'll do my best.
Anila wrote: "Hmm. I took AP Chem last year and got a 5 on the AP test, so maybe I'll be able to help out in here. Not sure how much I remember, but I'll do my best.Just a quick word of encouragement: If yo..."
Great words of encouragement!
Steve wrote: "Great explanation, Graziella."
thanks Steve :)
And I want to add too that when you are asked to write the electron configuration of a atom. Instead of drawing the an energy-level diagram you would write it like so:
Fe:
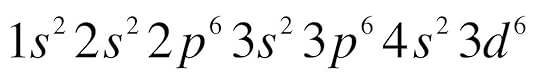
so you are just writing the orbital and the number of electrons found in that orbital would be placed as an exponent.
 I don't like chemistry :( My teacher doesn't believe in 'teaching'; he thinks we can just learn magically by reading our textbooks :/
I don't like chemistry :( My teacher doesn't believe in 'teaching'; he thinks we can just learn magically by reading our textbooks :/
 GSGS {Jizzy} wrote: "I don't like chemistry :( My teacher doesn't believe in 'teaching'; he thinks we can just learn magically by reading our textbooks :/"
GSGS {Jizzy} wrote: "I don't like chemistry :( My teacher doesn't believe in 'teaching'; he thinks we can just learn magically by reading our textbooks :/"That's exactly the same with my maths teacher!!! UGH so stressed...
But science, yeah... I know a bit about chemistry ^-^
 Andrea (Dru) wrote: "I saw that last year! hate it!!"
Andrea (Dru) wrote: "I saw that last year! hate it!!"Its hard to remember the order in which the energy level's fill...
I can do well with a textbook...it all depends on your learning styles too.
ohh maybe we can get someone else to go over Redox Reactions and balancing them? (I have yet to master it completely)
 If it's on the right, you start at +1 with the first family, and you just go like that until the fourth family, where it's plus or minus 4, and then from that you start -3,-2,-1..
If it's on the right, you start at +1 with the first family, and you just go like that until the fourth family, where it's plus or minus 4, and then from that you start -3,-2,-1..So it's like (reading left to right) +1, +2, +3, +or-4, -3,-2,-1,0
 Kaitlyn Analyssa wrote: "Ok i think i'm understading but i have another question, when u write chemical formulas how do u know what the charge is?"
Kaitlyn Analyssa wrote: "Ok i think i'm understading but i have another question, when u write chemical formulas how do u know what the charge is?"That's good Kaitlyn and How do you mean by the charge? are you talking about the valence electron and whether or not it is negative and or positive?
 I believe by family Karishma means Group which is then always going to be the column.
I believe by family Karishma means Group which is then always going to be the column.I'll make up a image give me a few minutes.
 Generally there is no shortcut to remember the charges for the elements in the green box (in image above) So you have to kind of just remember the common element's charge.
Generally there is no shortcut to remember the charges for the elements in the green box (in image above) So you have to kind of just remember the common element's charge. Fe is +3 while Co(Coblat) has +2 and Rh has a +3 charge ect...
 Kaitlyn Analyssa wrote: "Karishma wrote: "If it's on the right, you start at +1 with the first family, and you just go like that until the fourth family, where it's plus or minus 4, and then from that you start -3,-2,-1..
Kaitlyn Analyssa wrote: "Karishma wrote: "If it's on the right, you start at +1 with the first family, and you just go like that until the fourth family, where it's plus or minus 4, and then from that you start -3,-2,-1....."
Yeah sorry, I meant group. Group, family, column, it's all the same thing. And like Graziella said, you just kind of have to remember the charges for transition metals
 DAYYUM! I had my Chemistry today! It was EPIC!...not in a battle/war sense...that'd make no sense..right?!
DAYYUM! I had my Chemistry today! It was EPIC!...not in a battle/war sense...that'd make no sense..right?!Okay..I'll stop now..
It went really well.
 @Seraph: lol...you just do. Keep reading it bout 10 times...and test yourself. It's usually the first TWO letters...
@Seraph: lol...you just do. Keep reading it bout 10 times...and test yourself. It's usually the first TWO letters...
 True, good method.
True, good method.Guys I don't have to present my Chemistry final :D thank you mexican grading system!
 GUYS I SRRSLY NEED HELP LIKE NOW!
GUYS I SRRSLY NEED HELP LIKE NOW!I NEED TO FIND THE COMMON ALLOY FOR CALCIUM AND ITS PGYSICAL PROPERTIES!!
LIKE DUDES IVE CHECKED EVERYWHERE AND I CNT FIND IT AND MY ASSIGNMENT IS DUE TOMOZ! HAHA TOMOZ
YES YES I KNOW I LEFT IT TO THE LAST MINUTE BUT I SRRSLY NEED HELP! PLZ HELP!
HELP HELP HELP HELP HELP HELP HELP
 ikr! ahrg its soo annoying stupid teachers give u the most gayest stuff that ur not even ganna use in LIFE! wen u grow up!
ikr! ahrg its soo annoying stupid teachers give u the most gayest stuff that ur not even ganna use in LIFE! wen u grow up!soz im jst stressed haha..ill jst keep googling xD
thanx anywayz tho xDDDD
 Scarlet wrote: "Can someone please explain moles?"
Scarlet wrote: "Can someone please explain moles?"Mole day! Lol all I know is the number is 6.02x10^23 and it's Avogadro's number. If I can find the link to my friend's mole day song, I'll try to link it to ya
 Ahhh I'm in the very last sections of this chemistry lab I have to do and I'm so confused! I tried looking it up online but I'm not sure if the answers are right...I need to turn this in VERY SOON so help would be extremely appreciated!
Ahhh I'm in the very last sections of this chemistry lab I have to do and I'm so confused! I tried looking it up online but I'm not sure if the answers are right...I need to turn this in VERY SOON so help would be extremely appreciated!Part III: Reaction between zinc and acetic acid
1.Write the balanced equation for the reaction between zinc and acetic acid.
2.Classify the reaction between zinc and acetic acid and explain, in general terms, what happens during this type of reaction.
3.Give an example of a type of element and a type of compound that are likely to participate in this type of reaction.
Part IV: Reaction between baking soda and vinegar
1.Two types of reaction occur when baking soda and vinegar combine. The first is a double replacement reaction. Write a balanced equation for this reaction.
2.What type do you think the second reaction is? Why?
 I was in my final year when I realize highschool chemistry help a lot during reagent preparations (a lot for HPLC and spectro)... my advice, don't take the shortcut by asking ppl, do it yourself.
I was in my final year when I realize highschool chemistry help a lot during reagent preparations (a lot for HPLC and spectro)... my advice, don't take the shortcut by asking ppl, do it yourself.Unlike biology, Chemistry is not to be messed with.
 I already finished that, and I wasnt trying to take a shortcut, I just didnt understand how to find out the answers and was wondering if someone could explain it to me. The explainations online didnt help much either so I wanted to see if anyone else knew how to solve them.
I already finished that, and I wasnt trying to take a shortcut, I just didnt understand how to find out the answers and was wondering if someone could explain it to me. The explainations online didnt help much either so I wanted to see if anyone else knew how to solve them.
 Okay. Please, I beg you, do NOT give me the electron configuration. I'm trying to find the OUTER-electron configuration for Arsenic.
Okay. Please, I beg you, do NOT give me the electron configuration. I'm trying to find the OUTER-electron configuration for Arsenic.This is what I think it is: 4s23d104p3.
But the outer-configuration is only for the sub levels and electrons in the same period as the element I'm trying to configure, though, right? So does that mean, since the d-block goes up a period, that it's not technically supposed to be in the answer?
I think I'm right... But I'm not sure....
And what are they asking of me if they're saying "Graph the general trends in the second ionization energy (IE2) of an element as a function of its atomic number, over the range Z=1-20. Label the minima and maxima on the graph with the appropriate element symbol."?

![Hanna[h] Cumberbatch (andthetheyburiedmemummble) | 805 comments](https://images.gr-assets.com/users/1403470362p1/4536256.jpg)




